Swedish surgical innovation granted CE marking – paving the way for European launch
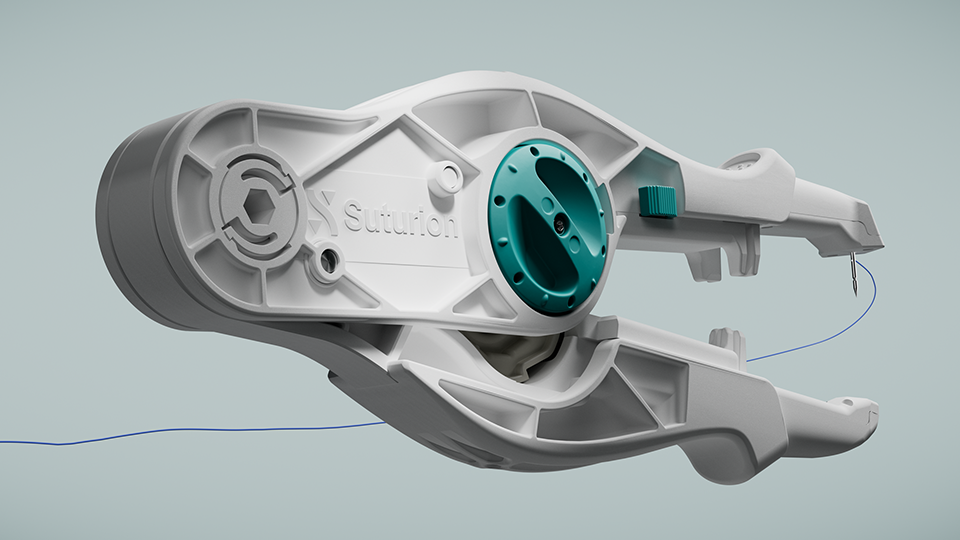
The Swedish MedTech company Suturion has been granted CE marking (MDR) for its groundbreaking surgical innovation SutureTOOL™. The use of the device – designed to enable standardized, efficient, and safe abdominal wall closure – has the potential to improve patient outcomes and significantly reduce healthcare costs across Europe.
The CE marking confirms that SutureTOOL™ meets the European Union’s high standards for safety and performance, opening the door for a broad European launch. This marks another major milestone for Suturion, following its U.S. debut earlier this year.
“Building on the FDA clearance in the United States, this certification represents the next major step in making our unique technology available to hospitals across Europe,” says Paan Hermansson, CEO of Suturion. “This approval has been long awaited. Studies have shown that applying the recommended small-bites technique can significantly reduce the number of complications, helping both to ease patient suffering and to save costs for healthcare systems. We are thrilled to finally be able to offer a tool that enables surgeons across Europe to use this technique more easily and consistently.”
Focus on key European countries
Suturion is now preparing to introduce SutureTOOL™ in several European countries, with an initial focus in the first phase on the United Kingdom, the Nordic region, and Benelux. To ensure broad clinical access, Suturion is partnering with well-established distributors, including Healthcare 21 (HC21) in the United Kingdom, and MBA Surgical Empowerment in Spain – both part of the Swedish life science group AddLife – as well as HCP Healthcarepartner in Austria. In markets where no distribution partner has yet been appointed, Suturion intends to work directly with hospitals until the most suitable local partner is identified.
Strengthening operations for international growth
To meet growing demand, Suturion is now preparing to scale up its production capacity and strengthen its organization. The company is also planning its largest funding round to date to support the next phase of commercialization and market expansion.
With regulatory approvals already in place in the U.S., the Middle East, and now the EU, and with Australia expected to follow soon, Suturion is establishing a strong global presence for SutureTOOL™.
A continued mission to improve surgical outcomes
Around one in three patients1-6 experience unnecessary complications after open abdominal surgery, often requiring additional procedures. SutureTOOL™ is a first-of-its-kind suturing device designed to support surgeons in performing abdominal wall closure in a standardized, efficient, and safe way – comparable to the precision of an advanced sewing machine.
By enabling the recommended small-bites suturing technique, the device helps reduce variation and lowers the risk of post-operative complications such as wound dehiscence and incisional hernia.
More information about Suturion can be found at: https://suturion.com/
References
1. Broach RB, et al. Ann Surg. 2017 Dec;266(6):946–951.
2. Deerenberg EB, et al. Lancet. 2015 Sep 26;386(10000):1254–1260.
3. Gonzalez M, et al. Langenbecks Arch Surg. 2023 Jan 20;408(1):50.
4. HART Collaborative. Br J Surg. 2022 Sep 9;109(10):943–950.
5. Millbourn D et al. Arch Surg 2009 Nov; 144(11): 1056-9.
6. Tolstrup MB et al. Ann Surg. 2017 Apr;265(4):821-826.
About CE marking
For medical devices, the CE mark confirms that the product has undergone a rigorous review process under the EU Medical Device Regulation (MDR 2017/745), including independent assessment by a Notified Body. In short, it assures users and patients that the device is safe, effective, and ready for use across Europe.
About Suturion
Suturion AB is a pioneering MedTech company based in Lund, Sweden, founded in 2018 by surgeon Gabriel Börner. The company’s groundbreaking innovation, SutureTOOL™, enables a standardized, efficient, and safe closure of the abdominal wall after open surgery. By empowering surgeons to perform fascia closure with precision, it significantly reduces the risk of post-operative complications while delivering substantial time and cost savings for healthcare systems. To learn more about Suturion and SutureTOOL™, visit www.suturion.com








