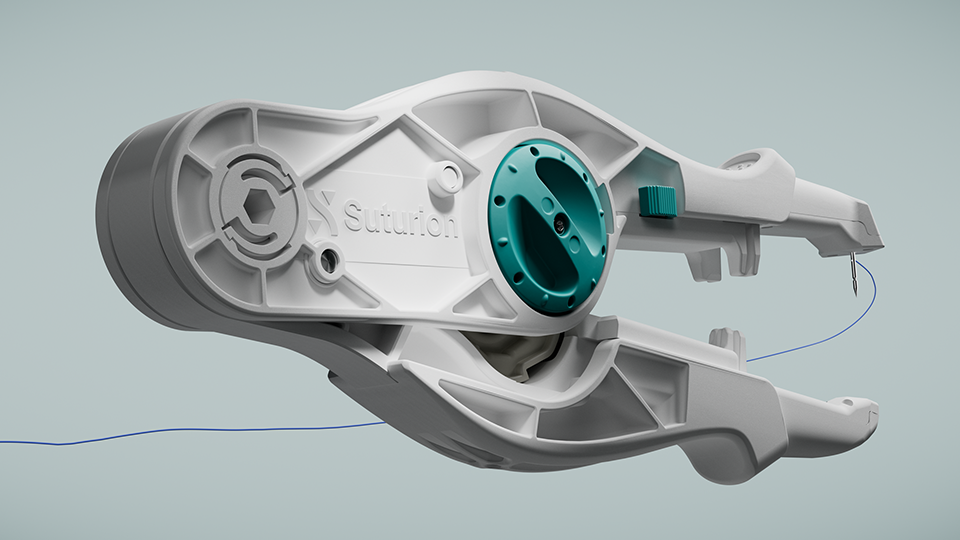
Clinical study confirms safety and efficiency of surgical innovation
The first clinical study of the Swedish suturing device SutureTOOL™ shows that surgeons can close the abdominal wall safely, efficiently, and with consistent quality – even after minimal training. Published in the scientific journal Hernia, the results highlight the device’s potential to reduce complications for patients worldwide.
Each year, surgeons perform around 30 million open abdominal surgeries worldwide1 – and up to one in three patients face serious complications.2 Surgical site infections, wound dehiscence, and incisional hernias are among the most common, often caused by inconsistent abdominal wall closure.1,3-7 Manual suturing remains the global standard, yet it is technically demanding, takes years to master, and is prone to variation even among experienced surgeons. This complexity not only increases the risk of complications but also limits how widely the recommended small-bites technique is applied.
SutureTOOL™ is the first device of its kind, developed by Swedish MedTech company Suturion, to standardize closure, make the small-bites technique easier to perform, and bring precision, efficiency and consistency to abdominal wall suturing – helping reduce the risk of complications and save valuable time in surgery. The newly published study – the first clinical evaluation of SutureTOOL™ – confirms exactly this, demonstrating that the device delivers safe and efficient closure.
Closure time cut by more than half
All participating surgeons successfully achieved the recommended suture-to-wound length ratio (SL/WL ≥ 4) on their first attempt. The average net closure time was only 7.4 minutes. In contrast, manual closure using the small bites technique typically takes 14–30 minutes, even for experienced surgeons. Notably, none of the surgeons in the study had prior experience with SutureTOOL™ and were only provided with written instructions and a training model.
The study, conducted at Helsingborg Hospital in Sweden between 2023 and 2024, included five surgeons and 38 patients with colorectal disease. No glove perforations or cases of burst abdomen were reported, and only one superficial wound infection occurred.
“The fact that all surgeons, with no previous experience, were able to follow the recommended closing technique from the very first case shows how effectively SutureTOOL™ standardizes a technique that can otherwise be difficult to execute correctly. That means more patients may receive a safe closure,” says Gabriel Börner, surgeon and founder of Suturion.
May significantly reduce healthcare costs
In addition to reducing time and complications, SutureTOOL™ may also contribute to lowering healthcare costs. A recent health economic study, conducted in collaboration with the independent Institute for Health Economics (IHE), showed that use of the device could reduce costs by 24 to 42 percent – depending on the market – compared to manual suturing. Two thirds of these savings can be realized within the first 90 days post-surgery. The study was based on data from Sweden, France, the UK, and the US.
Lund-based company taking on a global challenge
SutureTOOL™, which is entirely unique in its kind, is the first product from Suturion. Earlier this year, the device received clearance from the U.S. Food and Drug Administration (FDA) and has now officially been launched in the U.S. market. A European launch is planned for later this year.
“We’ve invested heavily in building a solid scientific foundation for SutureTOOL™ – both preclinical and now clinical. That makes this not just a unique innovation, but an evidence-based solution that can truly reduce patient suffering while helping healthcare systems save time and resources,” says Paan Hermansson, CEO of Suturion.
The clinical study was published in Hernia, June 2025. Read the full study here.
The health economic study was also published in Hernia, January 2025. Read the full study here.
References
1. HART Collaborative. Incisional hernia following colorectal cancer surgery according to suture technique: Hughes Abdominal Repair Randomized Trial (HART). Br J Surg. 2022 Sep 9;109(10):943-950.
2. Carney MJ et al. Trends in open abdominal surgery in the United States Observations from 9,950,759 discharges using the 2009-2013 National Inpatient Sample (NIS) datasets, Am J Surgery 2017, 214 (2); 287-292.
3. Millbourn D et al. Effect of stitch length on wound complications after closure of midline incisions: a randomized controlled trial. Arch Surg 2009; 144(11): 1056-9.
4. Broach RB et al. Randomized Controlled Trial of Two Alcohol-based Preparations for Surgical Site Antisepsis in Colorectal Surgery. Ann Surg. 2017 Dec;266(6):946-951.
5. Tolstrup MB et al. Reduced Rate of Dehiscence After Implementation of a Standardized Fascial Closure Technique in Patients Undergoing Emergency Laparotomy. Ann Surg. 2017 Apr;265(4):821-826.
6. Gonzalez M et al. Fascial dehiscence: predictable complication? Development and validation of a risk model: a retrospective cohort study. Langenbecks Arch Surg. 2023 Jan 20;408(1):50.
7. Deerenberg EB et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet. 2015 Sep 26;386(10000):1254-1260.