Swedish surgical innovation now launched in the U.S. – improves patient outcomes and reduces healthcare costs
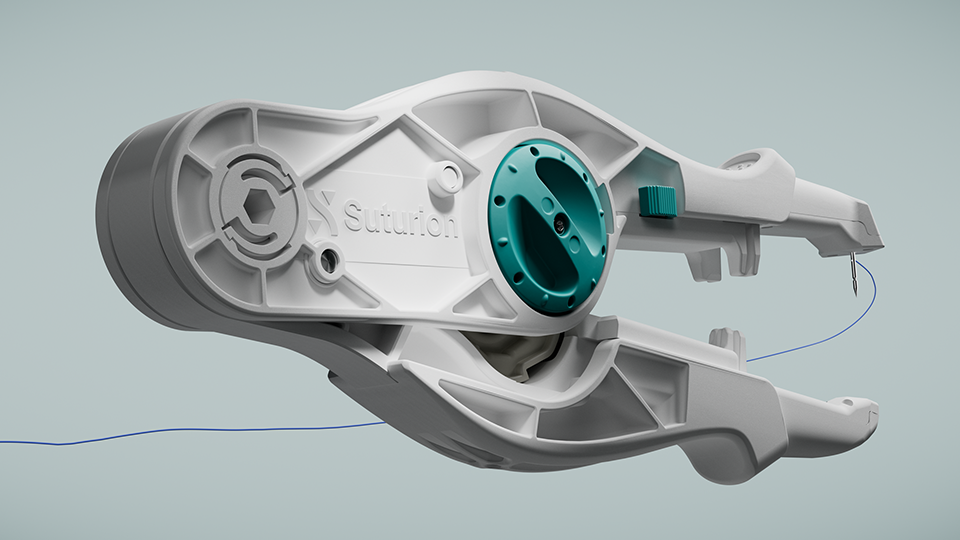
After more than 12 years of development and extensive testing, Swedish MedTech company Suturion is now launching its groundbreaking medical device SutureTOOL™ on the U.S. market. It is now officially confirmed that W. L. Gore & Associates Inc. will be the exclusive distributor.
Every year, around 30 million patients worldwide undergo open abdominal surgery, and more than 20 million cesarian sections are performed.1-3 Complications are common after laparotomy, often due to inadequate abdominal wall closure.1,4-8 These complications often result in long hospital stays, delayed recovery and additional surgeries, placing a heavy burden on both patients and the healthcare system.
This is the reason SutureTOOL™ was invented by Swedish surgeon Gabriel Börner – an easy-to-use surgical suturing device that brings the precision of an advanced sewing machine to abdominal wall closure. By enabling the recommended suturing technique in a standardized, efficient and safe way, it has the potential to improve surgical outcomes and reduce healthcare costs.
– This launch marks the greatest milestone in Suturion’s history to date, and we’re thrilled that SutureTOOL™ is finally available. This is a groundbreaking innovation with the potential to improve quality of patient care and safety, support surgeons in their daily work, and reduce the burden on healthcare systems globally, says Paan Hermansson, CEO of Suturion.
New U.S. Distributor Partner Selected
To support its U.S. market entry, Suturion has entered into a distribution partnership with the medical products business of U.S.-based W. L. Gore & Associates (Gore), a global material science company that develops innovative products across diverse industries. As the exclusive distributor of SutureTOOL™ in the United States, Gore will play a key role in accelerating adoption and expanding access the U.S. healthcare market.
Funding round supports expansion
Suturion is currently raising capital in its fourth funding round to support the next phase of international expansion.
1. HART Collaborative. Incisional hernia following colorectal cancer surgery according to suture technique: Hughes Abdominal Repair Randomized Trial (HART). Br J Surg. 2022 Sep 9;109(10):943-950.
2. Carney MJ et al. Trends in open abdominal surgery in the United States Observations from 9,950,759 discharges using the 2009-2013 National Inpatient Sample (NIS) datasets, Am J Surgery 2017, 214 (2); 287-292.
3. Molina G et al. Relationship Between Cesarean Delivery Rate and Maternal and Neonatal Mortality. JAMA. 2015 Dec 1;314(21):2263-70.
4. Millbourn D et al. Effect of stitch length on wound complications after closure of midline incisions: a randomized controlled trial. Arch Surg 2009; 144(11): 1056-9.
5. Broach RB et al. Randomized Controlled Trial of Two Alcohol-based Preparations for Surgical Site Antisepsis in Colorectal Surgery. Ann Surg. 2017 Dec;266(6):946-951.
6. Tolstrup MB et al. Reduced Rate of Dehiscence After Implementation of a Standardized Fascial Closure Technique in Patients Undergoing Emergency Laparotomy. Ann Surg. 2017 Apr;265(4):821-826.
7. Gonzalez M et al. Fascial dehiscence: predictable complication? Development and validation of a risk model: a retrospective cohort study. Langenbecks Arch Surg. 2023 Jan 20;408(1):50.
8. Deerenberg EB et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet. 2015 Sep 26;386(10000):1254-1260.